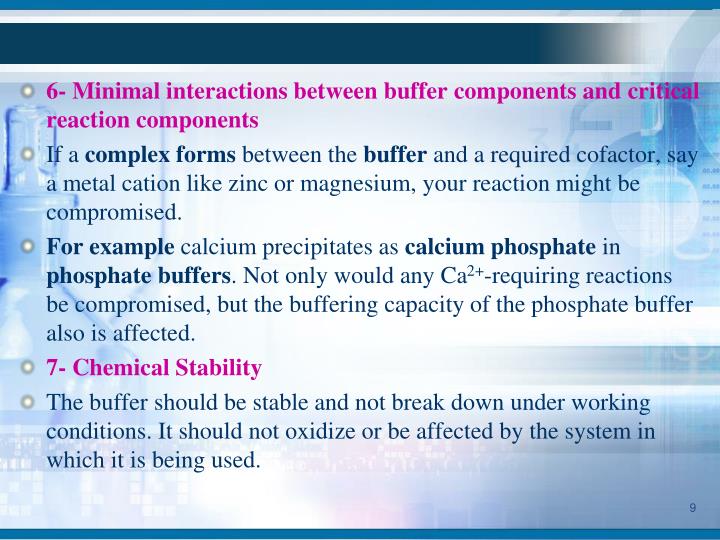
Biological Buffer System Ppt . Buffer + h+ h+ buffer. this document provides an overview of buffers in biological systems. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. It discusses how biological buffers help. Buffers • allow biological fluids to maintain relatively constant ph despite additions of acids or bases. A buffer is a solution that resists a significant change. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. buffers • biological systems use buffers to maintain ph. 2 buffers allow biological fluids to. you should be aware that buffers play a critical role in almost all biochemical systems.
from www.slideserve.com
this document provides an overview of buffers in biological systems. 2 buffers allow biological fluids to. It discusses how biological buffers help. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. you should be aware that buffers play a critical role in almost all biochemical systems. A buffer is a solution that resists a significant change. Buffers • allow biological fluids to maintain relatively constant ph despite additions of acids or bases. Buffer + h+ h+ buffer. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffers • biological systems use buffers to maintain ph.
PPT Buffers of Biological & Clinical Significance PowerPoint
Biological Buffer System Ppt 2 buffers allow biological fluids to. It discusses how biological buffers help. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. Buffers • allow biological fluids to maintain relatively constant ph despite additions of acids or bases. buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change. Buffer + h+ h+ buffer. 2 buffers allow biological fluids to. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. this document provides an overview of buffers in biological systems.
From exoxulnnf.blob.core.windows.net
Biological Roles Of Buffers at Dana Wild blog Biological Buffer System Ppt Buffer + h+ h+ buffer. 2 buffers allow biological fluids to. this document provides an overview of buffers in biological systems. It discusses how biological buffers help. you should be aware that buffers play a critical role in almost all biochemical systems. A buffer is a solution that resists a significant change. Buffers • allow biological fluids to. Biological Buffer System Ppt.
From www.vrogue.co
Ppt Buffers Of Biological Clinical Significance Power vrogue.co Biological Buffer System Ppt the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. A buffer is a solution that resists a significant change. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. Buffer + h+. Biological Buffer System Ppt.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffer System Ppt buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change. 2 buffers allow biological fluids to. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. It discusses how biological buffers help. Buffers • allow biological fluids to maintain. Biological Buffer System Ppt.
From ppt-online.org
Solutions. Acidbase equilibrium in biological systems презентация онлайн Biological Buffer System Ppt 2 buffers allow biological fluids to. buffers • biological systems use buffers to maintain ph. It discusses how biological buffers help. this document provides an overview of buffers in biological systems. A buffer is a solution that resists a significant change. Buffer + h+ h+ buffer. buffer is a mixture of weak acid and salt of conjugate. Biological Buffer System Ppt.
From ppt-online.org
Solutions. Acidbase equilibrium in biological systems презентация онлайн Biological Buffer System Ppt this document provides an overview of buffers in biological systems. 2 buffers allow biological fluids to. Buffer + h+ h+ buffer. A buffer is a solution that resists a significant change. you should be aware that buffers play a critical role in almost all biochemical systems. It discusses how biological buffers help. buffers • biological systems use. Biological Buffer System Ppt.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Biological Buffer System Ppt this document provides an overview of buffers in biological systems. It discusses how biological buffers help. you should be aware that buffers play a critical role in almost all biochemical systems. A buffer is a solution that resists a significant change. Buffers • allow biological fluids to maintain relatively constant ph despite additions of acids or bases. Buffer. Biological Buffer System Ppt.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffer System Ppt It discusses how biological buffers help. you should be aware that buffers play a critical role in almost all biochemical systems. A buffer is a solution that resists a significant change. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. buffers. Biological Buffer System Ppt.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffer System Ppt A buffer is a solution that resists a significant change. buffers • biological systems use buffers to maintain ph. this document provides an overview of buffers in biological systems. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffer is a mixture of weak acid. Biological Buffer System Ppt.
From www.youtube.com
buffer isotonic solution buffer capacity buffers in pharmaceutical Biological Buffer System Ppt this document provides an overview of buffers in biological systems. A buffer is a solution that resists a significant change. Buffer + h+ h+ buffer. 2 buffers allow biological fluids to. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. It discusses. Biological Buffer System Ppt.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Biological Buffer System Ppt It discusses how biological buffers help. you should be aware that buffers play a critical role in almost all biochemical systems. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. the purpose of a buffer in a biological system is to. Biological Buffer System Ppt.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint Biological Buffer System Ppt buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change. you should be aware that buffers play a critical role in almost all biochemical systems. It discusses how biological buffers help. Buffers • allow biological fluids to maintain relatively constant ph despite additions of acids or bases. this. Biological Buffer System Ppt.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson Biological Buffer System Ppt buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition of acid or base. buffers • biological systems use buffers to maintain ph. It discusses how biological buffers help. you should be aware that buffers play a critical role in almost all biochemical systems. this. Biological Buffer System Ppt.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Biological Buffer System Ppt this document provides an overview of buffers in biological systems. 2 buffers allow biological fluids to. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffers • biological systems use buffers to maintain ph. It discusses how biological buffers help. Buffer + h+ h+ buffer. . Biological Buffer System Ppt.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer Biological Buffer System Ppt It discusses how biological buffers help. buffers • biological systems use buffers to maintain ph. you should be aware that buffers play a critical role in almost all biochemical systems. 2 buffers allow biological fluids to. buffer is a mixture of weak acid and salt of conjugate base that resist the change in ph upon the addition. Biological Buffer System Ppt.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffer System Ppt you should be aware that buffers play a critical role in almost all biochemical systems. this document provides an overview of buffers in biological systems. It discusses how biological buffers help. Buffer + h+ h+ buffer. buffers • biological systems use buffers to maintain ph. buffer is a mixture of weak acid and salt of conjugate. Biological Buffer System Ppt.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffer System Ppt It discusses how biological buffers help. 2 buffers allow biological fluids to. buffers • biological systems use buffers to maintain ph. Buffer + h+ h+ buffer. A buffer is a solution that resists a significant change. this document provides an overview of buffers in biological systems. the purpose of a buffer in a biological system is to. Biological Buffer System Ppt.
From www.slideshare.net
Buffer system Biological Buffer System Ppt you should be aware that buffers play a critical role in almost all biochemical systems. buffers • biological systems use buffers to maintain ph. Buffer + h+ h+ buffer. 2 buffers allow biological fluids to. Buffers • allow biological fluids to maintain relatively constant ph despite additions of acids or bases. A buffer is a solution that resists. Biological Buffer System Ppt.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer Biological Buffer System Ppt you should be aware that buffers play a critical role in almost all biochemical systems. It discusses how biological buffers help. Buffer + h+ h+ buffer. buffers • biological systems use buffers to maintain ph. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. this. Biological Buffer System Ppt.